Dynamic differential scanning calorimetry (DSC) is the most commonly used method for thermal analysis because of its versatility and high conclusiveness of the results. Licharz has used DSC for about 25 years for the quality control of our LiNNOTAM products because their high sensitivity makes even small changes in the material easy to spot.
When developing new Licharz products DSC enables us to immediately identify envisaged modifications regarding morphology, crystallinity, glass transformation temperature, melting point or the chain length of the polymers.
DSC functionality
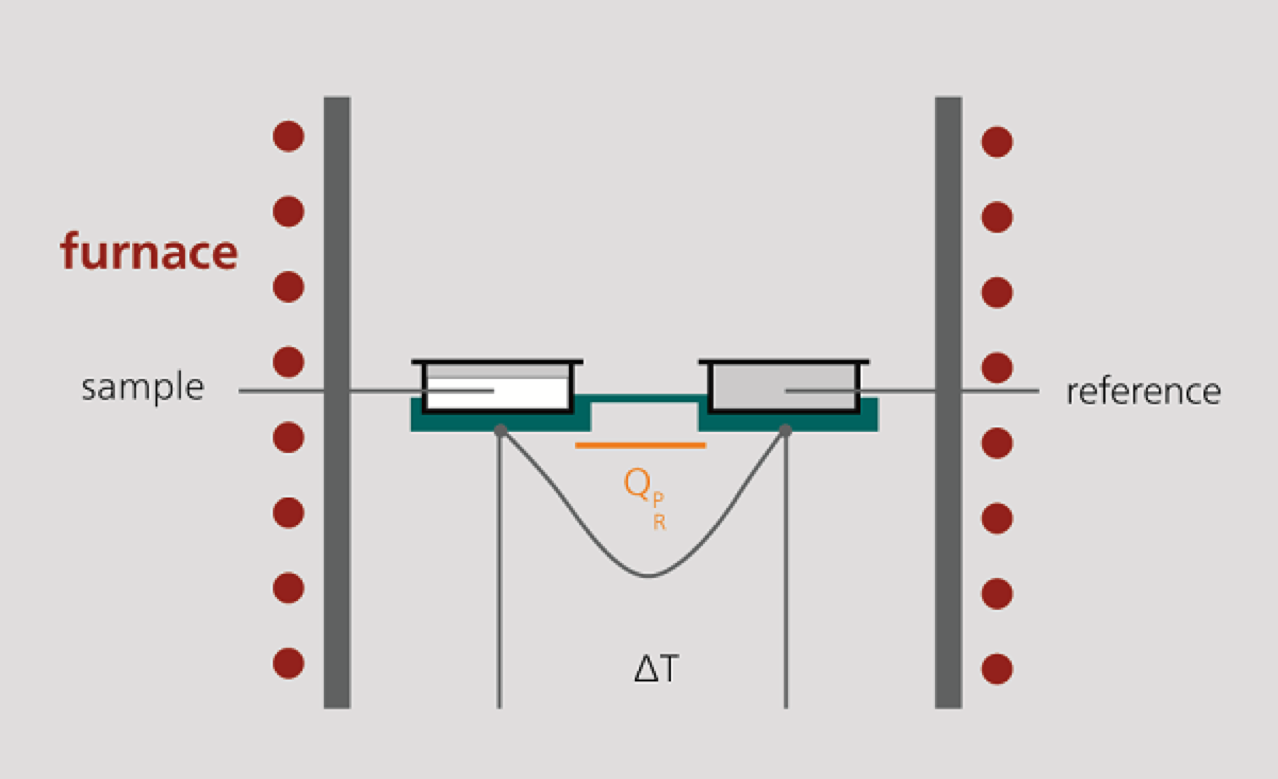
1. Measuring cell design
The DSC measuring cell consists of a furnace and an integrated sensor with corresponding shelves for the sample and reference trays. The sensor surfaces are connected to thermocouples or even form part of the thermocouple. In this way, both the temperature difference between sample side and reference side (DSC signal) and the absolute temperature of the sample side and reference side can be measured.
2. Heating the samples
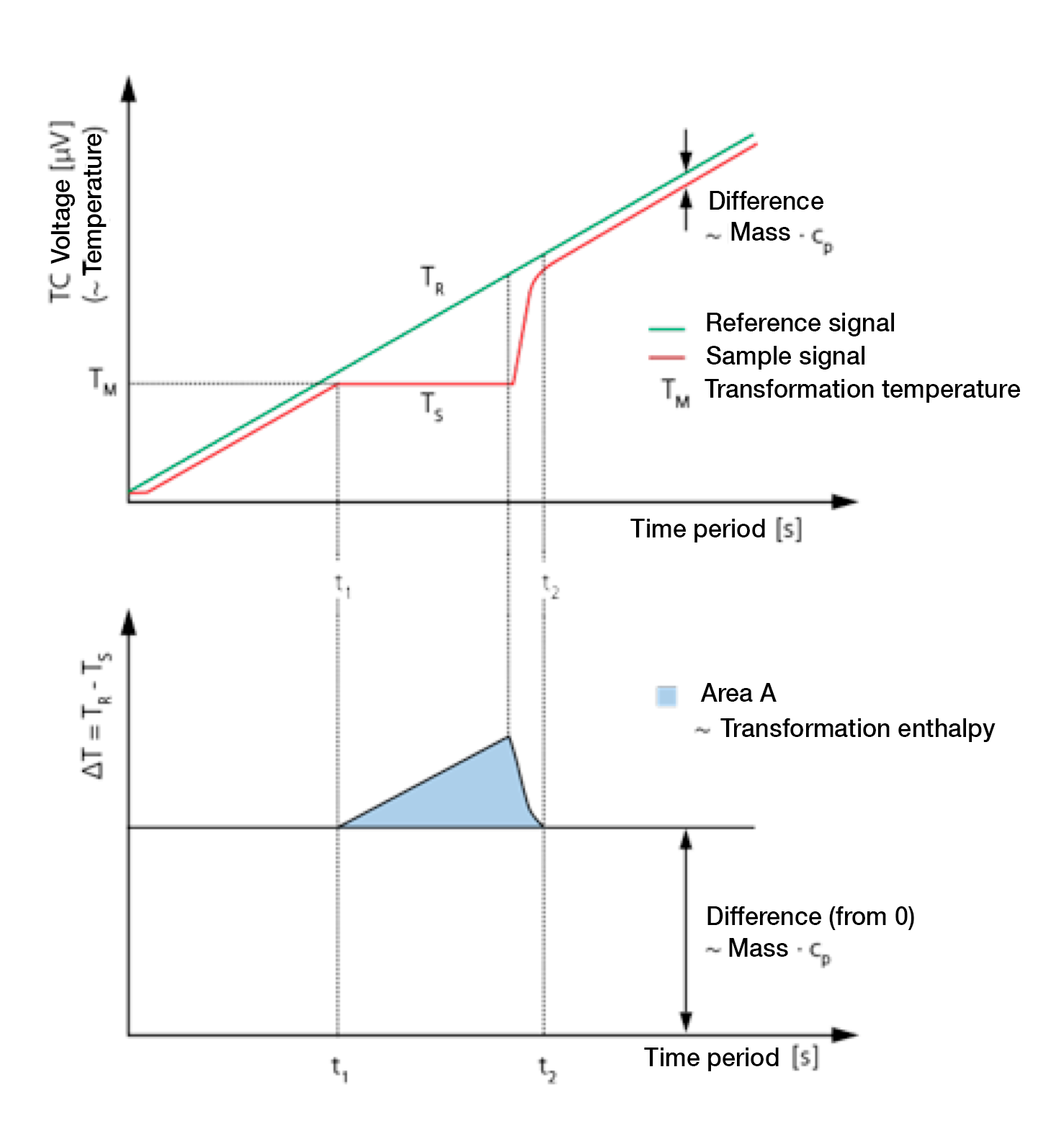
During the heating of a DSC measuring cell, the reference side (usually with an empty tray) normally warms up faster than the sample side due to the heat capacity (cp) of the sample. This means that the reference temperature (TR, green) rises slightly faster than the sample temperature (Ts, red). When heating at a constant rate both curves develop in parallel with each other until a sample reaction occurs.
3. Melting process
In the example given, (see figure), the sample begins to melt at time t1. During this time the temperature of the sample does not change. The temperature of the reference side, however, remains unaffected and continues to increase linearly. Once melting is completed the sample temperature once again increases and displays a linear slope from the time t2.
4. Analysis of the probe
To examine the specific material properties and the thermal history of a sample, typically the sample is twice heated above the melting point at a defined heating rate and cooled once. In this case the following parameters were determined:
- In the 1st heating period the melting point was determined (here 231.7° C). From the area of the melting peak (here 91.18J/g) a degree of crystallization of 48% was determined in this example.
- In the 2nd heating period it was possible to determine the specific mineral parameters and provide an indication of possible bi- or multimodal crystallite in the structure.
- During cooling the crystallization temperature was determined (here 166.2 ° C).
In the example given it was possible to determine further characteristics of the material-specific additives present that are not addressed in detail here.